Over the past year fda designed and developed the global unique device identification database gudid to prepare forthe implementation of the udi final rule.
Fda global unique device identification database.
Proposed rule requiring most medical devices distributed in the united states to carry a udi.
The fda is establishing the unique device identification system to adequately identify devices sold in the u s from manufacturing through distribution to patient use.
The fda udi help desk will email you the gudid new account request document in a fillable pdf format.
Fda established a set of compliance dates by device classification for compliance with required labeling and data submission to the global unique device identification database gudid under the.
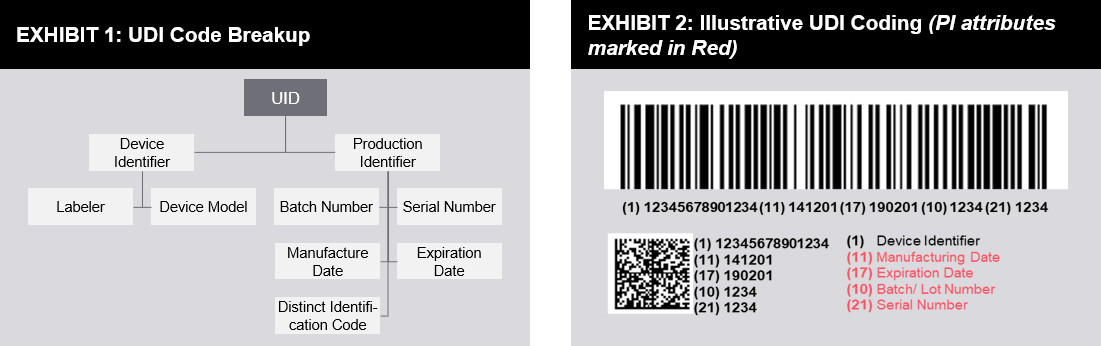
The unique device identifier udi should be created and maintained by device labelers based on global device identification standards managed by fda accredited issuing agencies 2 3.
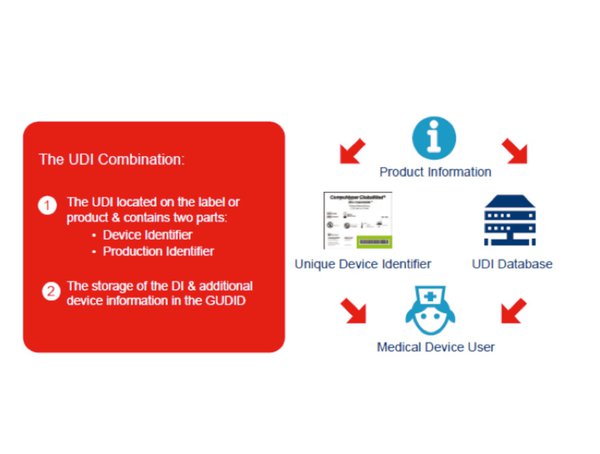
The global unique device identification database gudid is a database administered by the fda that will serve as a reference catalog for every device with a unique device identifier udi.
This document is primarily intended for device labelers and provides information necessary for submitting data to the global unique device identification database gudid.
On june 26 2014 fda issued the global unique device identification database gudid.
Global unique device identification database gudid gudid guidance.
A draft version of this.
The global unique device identification database gudid contains key device identification information submitted to the fda about medical devices that have unique device identifiers udi.